Abstract
Introduction: One of the mainstays of chemotherapy in acute myeloid leukemia (AML), other than acute promyelocytic leukemia, is induction with a goal to achieve morphological complete remission (CR) as evident by less than 5% blasts. Post remission strategies then focus on either consolidation chemotherapy or allogeneic bone marrow transplantation (aBMT). However, not all patients by this remission criterion achieve long term remission and a subset of patients relapse. This relapse originates from further expansion chemoresistant clones. Detection of minimal residual disease (MRD) following chemotherapy, early in the course of treatment, is highly predictive of outcome and offers a window of opportunity to intensify treatment and prevent relapse. Here, we describe assessment of immunophenotypic MRD using a 10-colour two tube assay and a "difference from normal" approach.
Methods: We accrued 280 consecutive patients of adult (>18 years) AML, other than with t(15;17), over a 30 month period after obtaining consent. All patients received "3+7" induction therapy with daunorubicin and cytarabine. If patients were in morphological CR at end of induction, they either received 3 courses of 12-18 gm/m2 high dose cytarabine (HiDAC) or aBMT if feasible.MRD testing was done at end of induction and if feasible, post 1st Cycle HiDac using a two tube 10 colour assay. (CD15, CD13, CD19, CD34, CD56, CD7, CD45, CD11b, HLA-DR, CD117, CD14, CD123, CD64, CD33, CD36 & CD38). A minimum of 1.6 million events were acquired per tube on a Navios flow cytometer. Identical panel was used at MRD time points as well as on the diagnostic sample. Analysis of MRD was done using Kaluza 1.3 by a difference from normal approach that focused on the development of progenitors to monocytes. Conventional karyotyping and FISH was done as per standard recommendations and patients were classified into favorable, intermediate and poor cytogenetic risk. The presence of FLT3 -ITD, NPM1 and CEBPA mutations was detected by a fragment length analysis based assay. Overall survival (OS) was calculated from date of diagnosis to time of last follow up or death. Relapse free survival (RFS) was calculated after achieving of 1st remission (CR) till relapse or death or last follow up if in CR. Results of the MRD assays, cytogenetic and molecular risk groups were analyzed for their impact on OS and DFS using Log Rank test of Kaplan-Meier survival analysis.
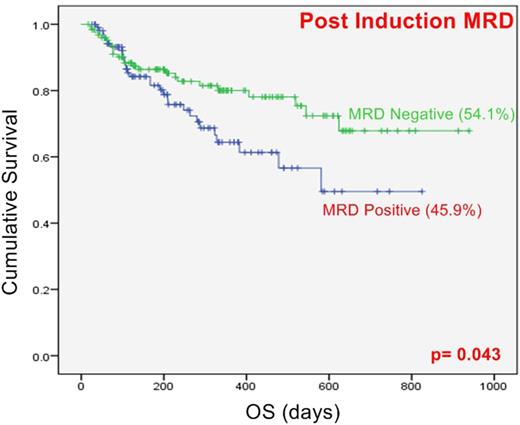
Results: A total of 280 AML patients were treated and followed up till maximum of 30 months period. Based on cytogenetics, 28.6% were classified as favorable risk whereas 61.4% and 10% were intermediate and poor risk respectively. FLT3-ITD, NPM1 and CEBPA mutations were harbored by 20.7%, 26.4% and 10.3% of patients respectively. The median follow up was of 9 months (Ranging from 17 days to 30 months). Of these, 35 had induction death and 59 had refractory disease. Median OS was 50.3% and RFS was 50.9%. Post induction MRD was assessed in 235 patients of which 108 (45.9%) had detectable residual disease (range 0.01-66%, median:1.38%). Post consolidation MRD was assessed in 98 patients of which 22 (22.4%) were MRD positive (range 0.002-7.7%, median: 0.05%). Favorable risk cytogenetics in cases excluding induction deaths was predictive of better OS than intermediate (p= 0.031) and poor risk (p=0.003) cytogenetic profiles but not RFS. Patients harboring MRD at the end of induction were associated with worse OS (p=0.04) but not RFS, whereas post consolidation positive MRD status did not show significant change in OS and RFS.
Conclusion: Our data is in agreement with other studies that determination of immunophenotypic MRD is extremely important in predicting outcome. AML MRD is a very useful guide for guiding post remission strategies in AML and should be incorporated into routine treatment algorithms.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal